
SL Paper 1
In which set do all the species contain more electrons than neutrons?
A. 14N, 16O, 11C
B. 14N, 16O, 11C4–
C. 14N3–, 16O2–, 11C
D. 14N3–, 16O2–, 11C4+
Markscheme
C
Examiners report
What is the relative atomic mass of an element with the following mass spectrum?

A. 24
B. 25
C. 26
D. 27
Markscheme
A
Examiners report
There were G2 comments about the mathematical ability required for this question and the format of the graph. The high difficulty index of 80% and satisfactory discrimination index of 0.42 would however indicate that it was accessible to candidates.
Which statement about the species \(^{{\text{63}}}{\text{C}}{{\text{u}}^{2 + }}\) and \(^{{\text{65}}}{\text{C}}{{\text{u}}^ + }\) is correct?
A. Both species have the same number of protons.
B. Both species have the same number of electrons.
C. Both species have the same number of neutrons.
D. Both species have the same electron arrangement.
Markscheme
A
Examiners report
What does \({}_{12}^{24}M{g^{2 + }}\) represent?
A. An ion with 12 protons and 24 neutrons
B. An ion with 14 protons and 24 neutrons
C. An ion with 12 protons and 12 neutrons
D. An ion with 12 protons and 22 neutrons
Markscheme
C
Examiners report
Which statement about the electromagnetic spectrum is correct?
A. Infrared light has a shorter wavelength than ultraviolet light.
B. Visible light has a shorter wavelength than ultraviolet light.
C. The frequency of visible light is higher than the frequency of infrared light.
D. The energy of infrared light is higher than the energy of visible light.
Markscheme
C
Examiners report
Which ion will show the least deflection in a mass spectrometer?
A. \(^{{\text{35}}}{\text{C}}{{\text{l}}^ + }\)
B. \(^{{\text{35}}}{\text{C}}{{\text{l}}^{2 + }}\)
C. \(^{{\text{35}}}{\text{C}}{{\text{l}}^{{\text{35}}}}{\text{C}}{{\text{l}}^ + }\)
D. \(^{{\text{35}}}{\text{C}}{{\text{l}}^{{\text{37}}}}{\text{C}}{{\text{l}}^ + }\)
Markscheme
D
Examiners report
We recognize that students might have found the molecular ions confusing. The question was answered correctly by 49.8% of the candidates and the question discriminated well (0.48). This is an example of a question set to discriminate the higher-grade candidates.
What is the composition of the nucleus of 26Mg?

Markscheme
D
Examiners report
The full electron configuration of an element is:
1s22s22p63s23p2
To which group and period does the element belong?
Markscheme
D
Examiners report
What is the correct number of each particle in an oxygen ion, \(^{{\text{18}}}{{\text{O}}^{2 - }}\)?

Markscheme
D
Examiners report
Two respondents commented on “oxygen ion” rather than “oxide ion”. The former was chosen when the paper was set to draw attention to the particular nature of the isotope in question.
What is the number of protons and the number of neutrons in 131I?
Markscheme
A
Examiners report
How many electrons does the ion \(_{{\text{15}}}^{{\text{31}}}{{\text{P}}^{3 - }}\) contain?
A. 12
B. 15
C. 16
D. 18
Markscheme
D
Examiners report
Which electron transition emits radiation of the longest wavelength?
Markscheme
C
Examiners report
Which is an isotope of 24Mg?
A. \(_{{\text{11}}}^{{\text{24}}}{\text{Na}}\)
B. \(_{{\text{21}}}^{{\text{24}}}{\text{M}}{{\text{g}}^{2 + }}\)
C. \(_{{\text{12}}}^{{\text{26}}}{\text{Mg}}\)
D. \(_{{\text{10}}}^{{\text{22}}}{\text{Ne}}\)
Markscheme
C
Examiners report
What is the name of the type of spectrum consisting only of specific wavelengths?
A. Electromagnetic
B. Continuous
C. Line
D. Mass
Markscheme
C
Examiners report
Which statements about the isotopes of chlorine, \(_{{\text{17}}}^{{\text{35}}}{\text{Cl}}\) and \(_{{\text{17}}}^{{\text{37}}}{\text{Cl}}\), are correct?
I. They have the same chemical properties.
II. They have the same atomic number.
III. They have the same physical properties.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
Which electron configuration is correct for the selenide ion, Se2−?
A. 1s2 2s2 2p6 3s2 3p6 4s2 4d10 4p4
B. 1s2 2s2 2p6 3s2 3p6 4s2 4d10 4p6
C. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
D. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Markscheme
D
Examiners report
Which statement about the numbers of protons, electrons and neutrons in an atom is always correct?
A. The number of neutrons minus the number of electrons is zero.
B. The number of protons plus the number of neutrons equals the number of electrons.
C. The number of protons equals the number of electrons.
D. The number of neutrons equals the number of protons.
Markscheme
C
Examiners report
One respondent suggested that neutral should have been inserted into the question for clarification purposes. However, neutrality is implied by the term atom in the question itself.
In the emission spectrum of the hydrogen atom, which electronic transition would produce a line in the ultraviolet region of the electromagnetic spectrum?
A. \(n = 1 \to n = 3\)
B. \(n = 3 \to n = 1\)
C. \(n = 3 \to n = 2\)
D. \(n = 10 \to n = 2\)
Markscheme
B
Examiners report
Which species have the same number of electrons?
I. \({{\text{S}}^{2 - }}\)
II. \({\text{C}}{{\text{l}}^ - }\)
III. Ne
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
Which diagram shows a pattern similar to the emission spectrum of hydrogen?
\(\xrightarrow[{{\text{Increasing wavelength}}}]{}\)
A. 
B. 
C. 
D. 
Markscheme
B
Examiners report
What are the numbers of neutrons and electrons in the iodine ion, \(^{{\text{125}}}{{\text{I}}^ + }\)?

Markscheme
B
Examiners report
Which is correct for the line emission spectrum for hydrogen?
A. Line M has a higher energy than line N.
B. Line N has a lower frequency than line M.
C. Line M has a longer wavelength than line N.
D. Lines converge at lower energy.
Markscheme
C
Examiners report
Some possible electron transitions in a hydrogen atom are shown below. Which letter represents the electron transition with the highest energy in the emission spectrum?

Markscheme
B
Examiners report
Which subatomic particles are located in the nucleus of an atom?
A. Protons and electrons
B. Neutrons and electrons
C. Protons and neutrons
D. Protons, neutrons and electrons
Markscheme
C
Examiners report
In the emission spectrum of hydrogen, which electronic transition would produce a line in the visible region of the electromagnetic spectrum?
A. \(n = 2 \to n = 1\)
B. \(n = 3 \to n = 2\)
C. \(n = 2 \to n = 3\)
D. \(n = \infty \to n = 1\)
Markscheme
B
Examiners report
One respondent suggested that guesswork may be required to answer this question. However, this question was based on the emission spectrum of hydrogen, which relates to AS 2.3.3 in the guide and in the Teacher‟s notes it is clearly stated that the ultraviolet, visible and infrared regions of the spectrum should be considered. The question itself was answered correctly by 58.93% of candidates.
Which statements are correct for silicon?
I. Its electron arrangement is 2,8,4.
II. It has four electrons in its highest occupied energy level.
III. In the solid state, each silicon atom is covalently bonded to four other silicon atoms in a tetrahedral arrangement.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
Which electron transition in the hydrogen atom emission spectrum emits radiation with the longest wavelength?
A. n = 2 → n = 1
B. n = 1 → n = 2
C. n = 4 → n = 1
D. n = 3 → n = 2
Markscheme
D
Examiners report
Which is the electron configuration of a chromium atom in the ground state?
A. [Ne]3s23p64s13d4
B. [Ar]3d3
C. 1s22s22p63s23p64s23d4
D. [Ar]4s13d5
Markscheme
D
Examiners report
What is the atomic number of a neutral atom which has 51 neutrons and 40 electrons?
A. 40
B. 51
C. 91
D. 131
Markscheme
A
Examiners report
Though this did not affect the correct answer, this question wrongly gave the number of neutrons, rather than the mass number, as 91. The lack of blank responses, the high difficulty index of 75% (remember the higher the number the more accessible the question) and the discrimination index of 0.40 all indicate that this error did not affect the validity of the question.
Which statement about the periodic table is correct?
A. The elements with atomic numbers 8, 16 and 34 have the same number of main energy levels.
B. The elements with atomic numbers 8, 9 and 10 have similar chemical properties.
C. The elements with atomic numbers 20, 21 and 22 are in the same group.
D. The elements with atomic numbers 20, 38 and 56 have the same number of electrons in their outer energy level.
Markscheme
D
Examiners report
Which statement about the electromagnetic spectrum is not correct?
A. The wavelength of ultraviolet radiation is shorter than infrared radiation.
B. The frequency of visible radiation is higher than the frequency of ultraviolet radiation.
C. The energy of infrared radiation is lower than the energy of ultraviolet radiation.
D. Wavelength is inversely proportional to frequency.
Markscheme
B
Examiners report
In one G2 comment it was stated that this question was more physics in nature. However, AS 2.3.1 states that candidates should be able to describe the electromagnetic spectrum (EMS) and in the corresponding TN for this AS, it is clearly indicated that variations in wavelength, frequency and energy in the UV, Vis and IR regions of the EMS should be known. 62% of candidates got the correct answer to the question, namely, B.
Which statement about the isotopes of nitrogen is correct?

Markscheme
C
Examiners report
A sample of zinc has the following composition:

What is the relative atomic mass of the zinc in this sample?
A. 64.5
B. 65.0
C. 65.9
D. 66.4
Markscheme
B
Examiners report
Another respondent stated that this question was very difficult without the use of a calculator. This was found not to be the case in fact for candidates as 73% got the correct answer, B.
Which is correct for the chromium isotope \({}_{24}^{53}{\rm{Cr}}\)?
A. 24 neutrons and 53 nucleonsB. 24 protons and 29 nucleons
C. 24 protons and 29 neutrons
D. 24 electrons and 53 neutrons
Markscheme
C
Examiners report
What is the total number of valence electrons in \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}{{\text{O}}^ - }\)?
A. 16
B. 22
C. 23
D. 24
Markscheme
D
Examiners report
Which shows the number of subatomic particles in 31P3−?

Markscheme
A
Examiners report
Which statement about the isotopes of an element is correct?
A. They have the same mass number.
B. They have a different atomic number.
C. They have the same chemical properties.
D. They are located in different places in the periodic table.
Markscheme
C
Examiners report
Ultraviolet radiation has a shorter wavelength than infrared radiation. How does the frequency and energy of ultraviolet radiation compare with infrared radiation?
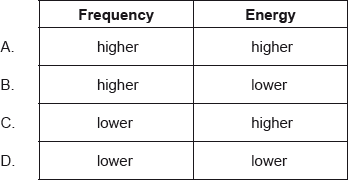
Markscheme
A
Examiners report
How many protons, neutrons and electrons are present in each atom of \(^{{\text{31}}}{\text{P}}\)?

Markscheme
B
Examiners report
Which is correct for the following regions of the electromagnetic spectrum?

Markscheme
A
Examiners report
Which are correct statements about the emission spectrum of hydrogen in the visible region?
I. The red line has a lower energy than the blue line.
II. The lines converge at longer wavelength.
III. The frequency of the blue line is greater than the frequency of the red line.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
Which species have the same electron arrangements?
I. \({{\text{O}}^{2 - }}\), \({{\text{F}}^ - }\), Ne
II. \({\text{L}}{{\text{i}}^ + }\), \({\text{N}}{{\text{a}}^ + }\), \({{\text{K}}^ + }\)
III. \({{\text{S}}^{2 - }}\), Ar, \({{\text{K}}^ + }\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
What does \(_{{\text{24}}}^{{\text{52}}}{\text{X}}\) represent?
A. An isotope of Te with 24 neutrons
B. An isotope of Te with 24 electrons
C. An isotope of Cr with 28 protons
D. An isotope of Cr with 28 neutrons
Markscheme
D
Examiners report
Which statement is correct for the ion \(_4^9{\text{B}}{{\text{e}}^{2 + }}\)?
A. The ion contains 15 subatomic particles in the nucleus.
B. The ion contains more protons than neutrons in the nucleus.
C. The ion has an electron arrangement of 2,2.
D. Most of the total volume of the ion is empty space.
Markscheme
D
Examiners report
Which statements about the chlorine free radical are correct?
I. It has 18 electrons.
II. It is an uncharged species.
III. It is formed by homolytic fission.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
Which ion will be deflected most in a mass spectrometer?
A. \(^{16}{{\text{O}}^ + }\)
B. \(^{16}{{\text{O}}^{2 + }}\)
C. \(^{18}{{\text{O}}^ + }\)
D. \(^{18}{{\text{O}}^{2 + }}\)
Markscheme
B
Examiners report
The diagram represents the emission spectrum of hydrogen. Groups of arrows are labelled W, X and Y.

Which statement is correct?
A. The arrows represent the transition of electrons to different energy levels when heat is supplied.
B. The arrows of W represent emission in the UV region.
C. The smallest arrow of X represents a violet line in the emission spectrum.
D. The arrows of Y represent emission of electromagnetic waves with higher energy than those represented by X and W.
Markscheme
B
Examiners report
What is the electron arrangement of the \({\text{M}}{{\text{g}}^{2 + }}\) ion?
A. 2,2
B. 2,8
C. 2,8,2
D. 2,8,8
Markscheme
B
Examiners report
What is the condensed electron configuration of the Fe2+ ion?
A. [Ar]3d6
B. [Ar]3d44s2
C. [Ar]3d54s1
D. [Ar]3d64s2
Markscheme
A
Examiners report
Which statements about the isotopes of an element are correct?
I. They have the same chemical properties.
II. They have different physical properties.
III. They have the same number of protons and electrons.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
Which species would be deflected most in a mass spectrometer?
A. \(^{{\text{24}}}{\text{M}}{{\text{g}}^{2 + }}\)
B. \(^{{\text{24}}}{\text{M}}{{\text{g}}^ + }\)
C. \(^{{\text{25}}}{\text{M}}{{\text{g}}^{2 + }}\)
D. \(^{{\text{25}}}{\text{M}}{{\text{g}}^ + }\)
Markscheme
A